Illustration (click to hide):
Project Description
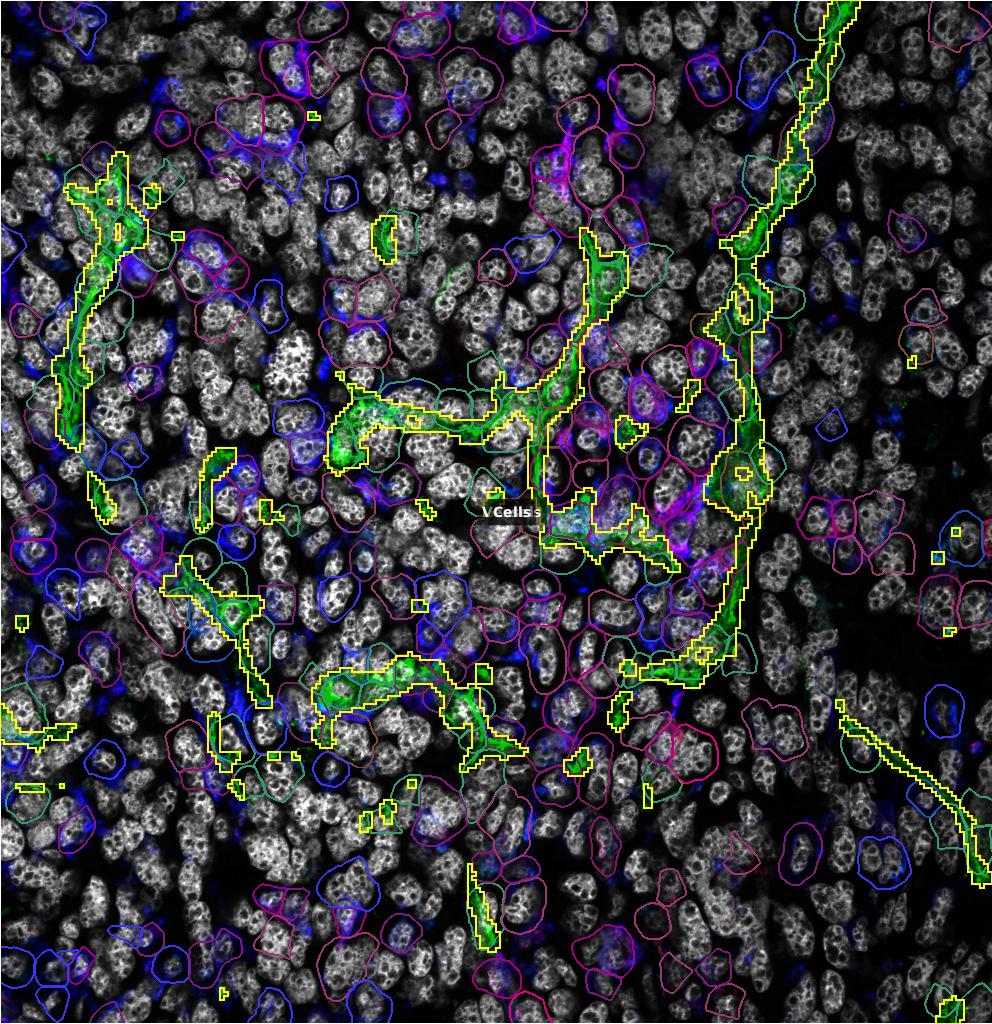
Accumulation of Tumor-Associated Macrophages (TAMs) is associated with enhanced tumor progression and poor survival in most solid cancers. TAMs are plastic cells exhibiting states and phenotypes spanning from anti-tumor/immunostimulating (sometimes referred to as M1-like) to pro-tumor/immunosuppressive (also denoted M2-like). In 2011, we showed that reprogramming of pro-metastatic TAMs located in the vicinity of tumor vessels (perivascular TAMs) induced vessel normalization and hampered metastatic dissemination. We have furthermore shown that TAM phenotypes may redirect metastatic dissemination towards the lymph nodes. Our published and preliminary data suggest the relevance of such TAMs also in human cancers, as their accumulation associates with increased malignancy and metastasis. We have recently pinpointed a novel pathway that regulates immunosuppressive and pro-metastatic TAMs. By utilizing this pathway and employing multiplex imaging, we investigate the role of perivascular TAMs and their phenotypes in regulating T cell trafficking, interaction, and development of high endothelial venules and metastatic dissemination. Furthermore, we investigate how TAM phenotypes regulate other perivascular cell phenotypes, including fibroblasts and pericytes, that control tumor growth and metastatic dissemination. In order to study cell-to-cell interaction in the vicinity of tumor blood vessels, we are now developing a workflow that can be used to answer the following questions in patient samples and murine tumor models:
-
Do anti-metastatic TAMs regulate leukocyte trafficking? If so, which leukocytes are affected?
-
Do anti-metastatic TAMs control perivascular fibroblast phenotypes?
-
Do anti-metastatic TAMs control pericyte phenotypes?
-
Do anti-metastatic TAMs control HEV formation and T cell trafficking?
-
Do normalized vessels induced by anti-metastatic TAMs associate with any of the above parameters?
Tags: TAMs
Project Information
-
BIIF Principal Investigators
- Agustin Corbat
External Authors
Charlotte Rolny, Charlotte Rolny, -
Date
2024-07-10 🠚 Current