Illustration (click to hide):
Project Description
Amoeboid cell migration, facilitated by the formation of dynamic plasma membrane blebs, is implicated in cancer cell migration, particularly in compressive environments such as those found in growing tumors. Blebbing is initiated by localized dissociations of the plasma membrane from the underlying actin cortex, and driven by increased cytosolic pressure, which can be cause by increased actomyosin-mediated contraction of the cytoskeletal network. Components of the coagulation cascade, including thrombin, are elevated in a variety of tumor environments, and we observe that thrombin stimulation of breast cancer cells leads to increased contraction of the cytoskeleton, which manifests as dynamic plasma membrane blebbing. Here we identify a functional role for the mechanosensitive calcium channel Piezo1 in attenuating thrombin-induced blebbing in breast cancer cells. We find that activation of Piezo1 via gentle compression of cells using a novel “Cell Press” device, or with an established Piezo1 agonist, attenuates blebbing and deactivates ERM proteins. ERM proteins crosslink the plasma membrane to the cytoskeleton, transmitting the contractile forces exerted by the cytoskeleton to the plasma membrane. Therefore, we propose a signaling mechanism whereby Piezo1 activation leads to an uncoupling of the contracting cytoskeleton from the plasma membrane; thus, relieving the cytosolic pressure required to drive dynamic bleb formation.
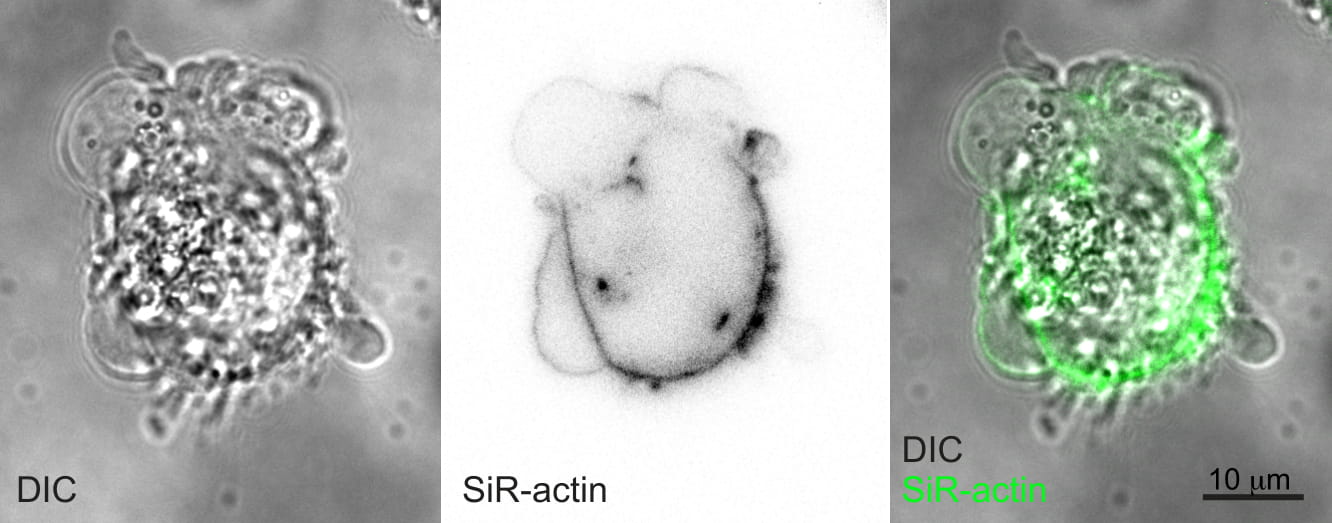
(Image: A blebbing MDA-MB-231 breast cancer cell imaged by differential interference contrast and fluorescence microscopy of SiR-actin labelled cytoskeleton)
The current manuscript is posted on bioRxiv
Project Information
-
BIIF Principal Investigators
- Christophe Avenel
External Authors
Paul O'Callaghan, Adam Engberg, Nikos Fatsis-Kavalopoulos, Gonzalo Sanchez, Olof Idevall-Hagren, and Johan Kreuger, Department of Medical Cell Biology, Uppsala University -
Date
2020-08-27 🠚 Current