Illustration (click to hide):
Project Description
For the last decades a concern has been raised that female fertility is declining – more than could be explained by the fact that we choose to have children later in life and possible genetic effects. Subfertility – and infertility – is a devastating experience for those who are affected and as the subject is also somewhat of a taboo – the numbers affected are most likely higher than perceived among the general public. In our environment, we are continuously exposed to a number of exogenous chemicals, originating from industries, agriculture and other. As many of these chemicals show persistence and are very bio-accumulative, they will concentrate higher up in the food-chain, in both wildlife and humans. Many of the chemicals are new – and have yet not been investigated regarding their full toxicological potential.
Perfluorononanoic acid (PFNA)
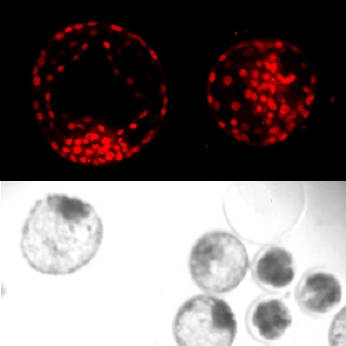
This project aim to further investigate perfluorononanoic acid (PFNA) and its effect on the early embryo development. This chemical is closely related to know toxic substances such as PFOS and PFOA, but is in contrary to those – little research has yet been done regarding PFNAs potential toxicological effects. We have used a bovine model, where we collect material from the slaughter-house that are normally disposed. We have exposed egg-cells to PFNA and followed the egg cells in the lab as they develop into very early embryos. The embryos are then investigated regarding morphology and with different staining helping us identifying differences in for example fat-metabolism of exposed embryos.
Code availability
https://github.com/BIIFSweden/Analysis-of-cow-embryos
Publications
Ida Hallberg, Jacklin Kjellgren, Sara Persson, Stefan Örn, Ylva Sjunnesson,
Perfluorononanoic acid (PFNA) alters lipid accumulation in bovine blastocysts after oocyte exposure during in vitro maturation
Reproductive Toxicology, Volume 84, 2019, Pages 1-8, ISSN 0890-6238, https://doi.org/10.1016/j.reprotox.2018.11.005. (https://www.sciencedirect.com/science/article/pii/S0890623818303423)
Abstract:
Perfluorononanoic acid (PFNA) is one of the perfluoroalkyl acids present in human tissues. In this study, effects on early embryo development after PFNA exposure were investigated using the bovine in vitro production system. Oocytes were exposed to PFNA during maturation in vitro (10 μg mL−1 and 0.1 μg mL−1), and then fertilized and cultured in parallel with control groups. Developmental parameters (cleavage, blastocyst formation) were followed and embryo quality evaluated (stage, grade). Embryos developed after exposure to 0.1 μg mL−1 were stained to distinguish nuclei, active mitochondria and neutral lipids. 10 μg mL−1 of PFNA had a severe negative effect on blastocyst formation (OR: 0.27 p < 0.05), an effect not observed at 0.1 μg mL−1. However, lipid droplet distribution was significantly altered in embryos exposed to 0.1 μg mL−1, suggesting a disturbance of lipid metabolism after exposure to sublethal levels of PFNA during oocyte maturation in vitro.
Keywords: Bovine; IVP; PFNA; Lipid droplet; Image analysis; Developmental toxicology; Endocrine disruption
Ida Hallberg, Sara Persson, Matts Olovsson, Mikaela Moberg, Petter Ranefall, Denise Laskowski, Pauliina Damdimopoulou, Marc-Andre Sirard, Joëlle Rüegg, Ylva C.B. Sjunnesson
Bovine oocyte exposure to perfluorohexane sulfonate (PFHxS) induces phenotypic, transcriptomic, and DNA methylation changes in resulting embryos in vitro
Reproductive Toxicology, Volume 109, 2022, Pages 19-30, ISSN 0890-6238, https://doi.org/10.1016/j.reprotox.2022.02.004. (https://www.sciencedirect.com/science/article/pii/S0890623822000235)
Abstract:
Knowledge on the effects of perfluorohexane sulfonate (PFHxS) on ovarian function is limited. In the current study, we investigated the sensitivity of oocytes to PFHxS during in vitro maturation (IVM), including consequences on embryo development at the morphological, transcriptomic, and epigenomic levels. Bovine cumulus-oocyte complexes (COCs) were exposed to PFHxS during 22 h IVM. Following fertilisation, developmental competence was recorded until day 8 of culture. Two experiments were conducted: 1) exposure of COCs to 0.01 µg mL-1 − 100 µg mL-1 PFHxS followed by confocal imaging to detect neutral lipids and nuclei, and 2) exposure of COCs to 0.1 µg mL-1 PFHxS followed by analysis of transcriptomic and DNA methylation changes in blastocysts. Decreased oocyte developmental competence was observed upon exposure to ≥ 40 µg mL-1 PFHxS and altered lipid distribution was observed in the blastocysts upon exposure to 1–10 µg mL-1 PFHxS (not observed at lower or higher concentrations). Transcriptomic data showed that genes affected by 0.1 µg mL-1 PFHxS were enriched for pathways related to increased synthesis and production of reactive oxygen species. Enrichment for peroxisome proliferator-activated receptor-γ and oestrogen pathways was also observed. Genes linked to DNA methylation changes were enriched for similar pathways. In conclusion, exposure of the bovine oocyte to PFHxS during the narrow window of IVM affected subsequent embryonic development, as reflected by morphological and molecular changes. This suggests that PFHxS interferes with the final nuclear and cytoplasmic maturation of the oocyte leading to decreased developmental competence to blastocyst stage.
Keywords: Per- and polyfluoroalkyl substances; PFAS; Female fertility; Bovine; IVP; Oocyte maturation; Embryo quality; Reproductive toxicity
Project Information
-
BIIF Principal Investigators
- Petter Ranefall
External Authors
Ylva Sjunnesson, Ida Hallberg, Kliniska Vetenskaper, avdelningen för reproduktion, SLU -
Date
2017-01-19 🠚 2019-12-12